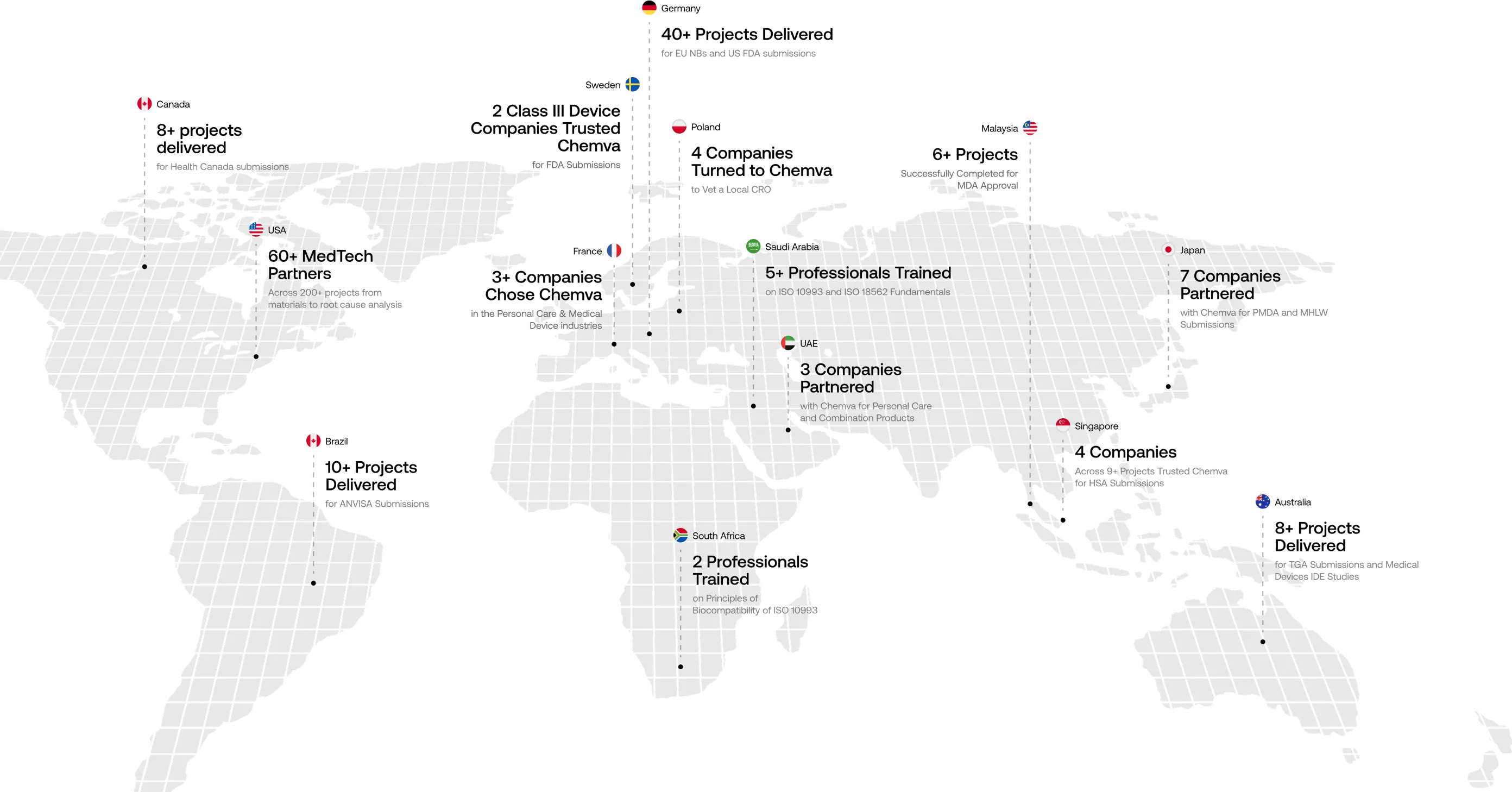
Your Global Scientific Partner
Trusted by Companies Across 15+ Countries
We craft customized preclinical strategies to help companies overcome complex biocompatibility challenges. At Chemva, our integrated scientific disciplines enable us to deliver globally unmatched consulting services. Our materials selection process, for instance, carefully evaluates potential biocompatibility issues and leachables challenges.
Swipe to scroll




