Science-Led
MedTech Solutions
The only global consulting firm merging scientific disciplines to solve complex challenges for your products — from early ideation and development to post-market support.
Book a free consultation
About Us
As a specialized MedTech consulting firm, we enable companies with materials, chemistry, and preclinical strategies. With over 500 projects in our experience.
Our experienced team assists with developing biological evaluation plan (BEP), design extractable/leachable studies, review protocols, screen contract research organization (CROs), represent you in meetings with regulatory agencies and CROs, review protocols, prepare toxicological risk assessments, and assemble a biological evaluation report (BER) — all with a focus on speed, quality, and transparency.
500
+
BEP, TRA, MRA, & BER.
With Our Unparalleled Expertise in ISO 10993 series, We Led Many Projects -- Expertise You Can Trust.
85
+
Global Clients, Our Partners.
Relying on Chemva’s integrated expertise in materials, chemistry, biocompatibility, and toxicology to accelerate projects.
150
+
Trained.
Educating Medical Device Innovators on Biocompatibility and Chemical Characterization Fundamentals.
9
+
Technical Committees.
Shaping the Future of ISO 10993 Standards as Industry Experts.
The Smarter Path to Regulatory Success
Others vs. Chemva®
This is some text inside of a div block.
Other Service Providers
Chemva®
Team Structure
Siloed experts working separately
Unified scientific team merging sciences for integrated consulting
Process
Redundant meetings; conflict of intertest with their internal labs; Repeat testing
Integrated approach with our clients from day one
Outcomes
Delays, upselling unnecessary tests, frustration, deficient submissions
Our clients' partner before labs, multi-approach strategies, clear submissions
Strategy
Reactive and fragmented
Purpose-driven and streamlined

Integrated Scientific Approach.
Optimized Materials Selection from the Start.
We take a holistic, lifecycle-based approach to material selection, considering maMaterial selection informed by real-world use and regulatory expectations.nufacturing and sterilization effects, storage and transport, extractables and leachables, biocompatibility, and material stability throughout intended use.

Expertise in Action
Trusted Delivery, Tangible Results
From precise testing to comprehensive digital reports, Chemva ensures every outcome is clear, reliable, and ready for regulatory submission. Our delivery means more than speed — it means confidence in every result.

Expertise in Action
Proven Partnership, Lasting Impact
Our clients count on Chemva to solve complex challenges. See how our science-driven solutions lead to safer products, faster approvals, and lasting trust.
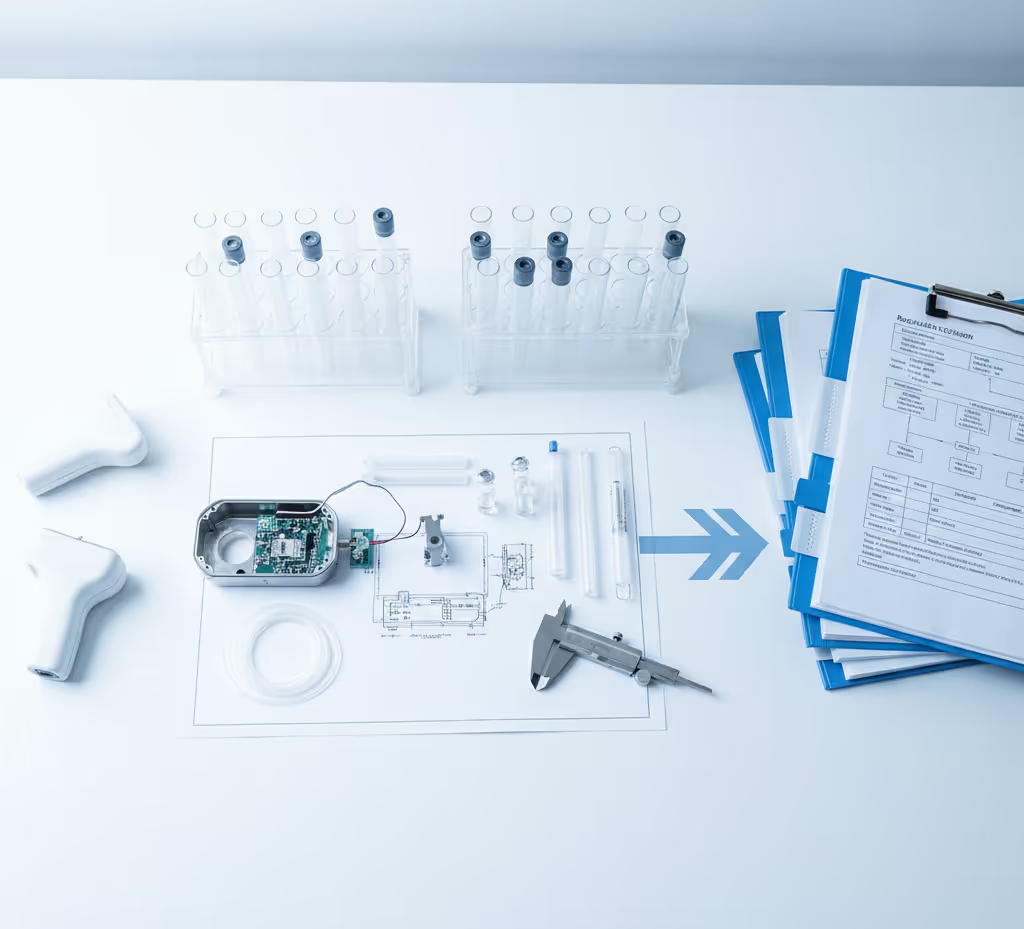
Expertise in Action
Proven Partnership, Lasting Impact
Our clients count on Chemva to solve complex challenges. See how our science-driven solutions lead to safer products, faster approvals, and lasting trust.
With You Every Step of the Way
Trusted by 85+ MedTech Innovators
Chemva delivers expert solutions, whether you need full-cycle support or help at a critical step.

Step 1
Product
Development
Development

Step 2
Manufacturing
Support
Support

Step 3
Preclinical
Biocompatibility
Biocompatibility

Step 4
Extractables/Leachables
(E&L)
(E&L)

Step 5
Post-Market Support
Why Chemva?
Because we care about your success as much as you do.
Here’s what makes us different.
Here’s what makes us different.

98
%
Client Retention Rate. Our clients don’t just come to us once — they come back. Trust, precision, and clarity are why.

Kinza F.
Business Devellopment Managet
Strategic Testing Support
Expert Support for
Certified Testing
Certified Testing
We are NOT a laboratory. We keep labs accountable for our clients.
We strive for you, so labs do not upsell unnecessary tests or you do not repeat testing.
We guide and support all aspects of product testing — from selecting qualified labs to reviewing biocompatibility, E&L, and toxicology protocol and data.
We strive for you, so labs do not upsell unnecessary tests or you do not repeat testing.
We guide and support all aspects of product testing — from selecting qualified labs to reviewing biocompatibility, E&L, and toxicology protocol and data.

Global-Ready Preclinical Strategies
We develop tailored testing strategies and review E&L and biocompatibility protocols to ensure your submission meets the expectations of global agencies, including FDA, NBs, Health Canada, MHRA, NMPA, TGA, HSA, and more.

Your Global Scientific Partner
Trusted by Companies Across 15+ Countries
We craft customized preclinical strategies to help companies overcome complex biocompatibility challenges. At Chemva, our integrated scientific disciplines enable us to deliver globally unmatched consulting services. Our materials selection process, for instance, carefully evaluates potential biocompatibility issues and leachables challenges.
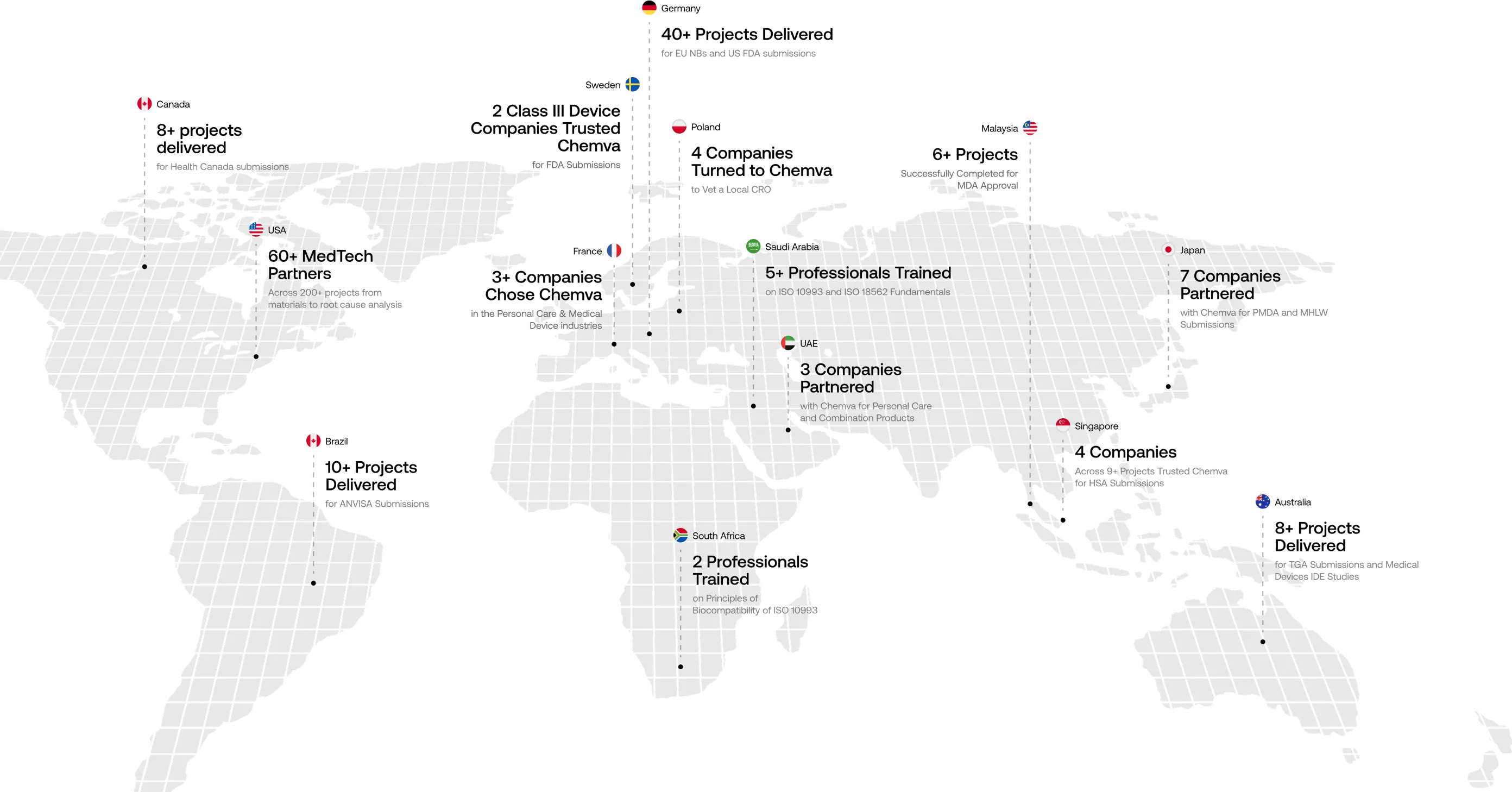
Swipe to scroll
.webp)
Proven Results
Real-World Insights From Complex Projects
Learn about our scientific approach that helps clients.
View all case studies
Testimonials
Leading Companies Trust Chemva to Navigate Complexity and Deliver Results
















.png)





